

Payers and policy makers should carefully examine study designs and assumptions and choose CEAs with greater validity and accuracy for evidence-based decision-making.
Differences in methodology across CEAs may lead to distinct results and conclusions. Consequently, results differed even among studies examining the same population and comparator within similar time intervals. Certain aspects of variability across models were problematic, including deviation from observed treatment utilization within trials and predicted long-term mortality risks for pembrolizumab higher than historical real-world NSCLC mortality data prior to the availability of pembrolizumab.

Differences were observed in health states (progression free, progressed disease, and death vs stable disease, progressed disease, death, and treatment discontinuation), modeling approaches (partitioned survival vs Markov), survival extrapolation/transition probability estimation, inclusion of additional costs to drug, disease management and adverse event costs (e.g., programmed death-ligand 1 testing, subsequent treatment, terminal care), treatment duration approaches (trial-based time on treatment vs treat to progression), utility sources (trial data vs literature), and utility analyses (time to death vs progression status). These publications covered regulatory-approved pembrolizumab NSCLC indications based on the following randomized clinical trials: KEYNOTE-010 (one publication), KEYNOTE-024 (six), KEYNOTE-042 (four), KEYNOTE-189 (two), and KEYNOTE-407 (one).

Modeling approaches, survival and cost estimation, and utility analyses were compared and evaluated. Fourteen studies were selected through searches of the PubMed database. We review published cost-effectiveness analyses (CEAs) of pembrolizumab as treatment for NSCLC and provide in-depth assessment of their methodologies.
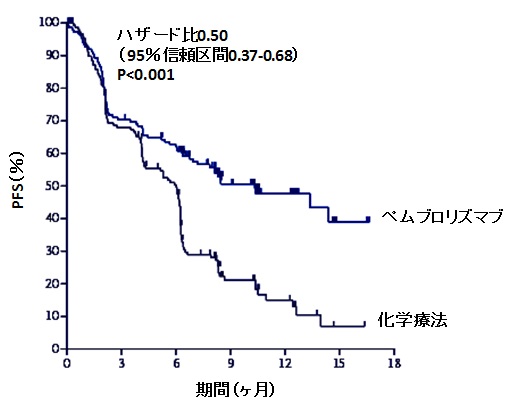
#KEYNOTE 042 TRIAL#
Meanwhile, in February the CHECKMATE-227 trial of BMS’ combination of Opdivo and Yervoy (ipilimumab) showed a significant benefit on PFS in first-line NSCLC patients with high TMB, regardless of PD-L1 expression.Pembrolizumab monotherapy or combination therapy is an approved treatment for various advanced non-small cell lung cancer (NSCLC) indications.
#KEYNOTE 042 PLUS#
Roche’s Impower150 study of PD-L1 inhibitor Tecentriq (atezolizumab) plus Avastin (bevacizumab) and chemotherapy has shown robust PFS data in previously-untreated non-squamous NSCLC patients, and AstraZeneca and BMS are both hoping to find a role for combinations pairing PD-1/PD-L1 inhibitors with CTLA4 inhibitors.ĪZ’s combination of Imfinzi (durvalumab) and tremelimumab missed the PFS endpoint in the MYSTIC trial last year but is continuing to an overall survival read-out. That’s not to say Merck’s position in first-line NSCLC is assured - particularly as the data from the latest trial is still under wraps. The difference in outcome between the Merck and BMS studies is interesting, and it’s not yet clear whether there is some difference between the drugs or simply the protocols used in the studies. Keytruda is already approved as a first-line monotherapy for NSCLC is patients with PD-L1 expression levels of 50% or more, so the new results mean it can now be used in more than two thirds of all patients presenting with this common form of cancer.
#KEYNOTE 042 FULL#
Specific results from the monotherapy study haven’t yet been revealed - Merck says it will present the numbers in full at a future medical meeting - and the trial will continue until data on progression-free survival (PFS) is mature. In January, the drug become the drug in the PD-1/PD-L1 class to improve overall survival when given in combination with chemotherapy in previously-untreated patients in the KEYNOTE-189 study. Merck’s head of R&D Roger Perlmutter said the trial provided further evidence that Keytruda is a “foundational treatment for NSCLC”, having shown consistent benefits on survival both as a monotherapy and in combination with chemotherapy. It’s worth noting that rival PD-1 inhibitor Opdivo from Bristol-Myers Squibb was unable to show a benefit as a monotherapy in that patient population, a finding which effectively handed the first-line NSCLC immunotherapy market to Merck’s drug. In the KEYNOTE-042 trial, PD-1 inhibitor Keytruda (pembrolizumab) improved overall survival compared to platinum hemotherapy when given on its own to ‘all-comer’ patients with locally advanced or metastatic NSCLC, including those with both squamous and non-squamous types and a full range of PD-L1 expression levels from 1% and above. Merck & Co is already the dominant force in first-line non-small cell lung cancer (NSCLC), and looks set to retain that title with another positive late-stage trial.


 0 kommentar(er)
0 kommentar(er)
